Research
Development and plasticity of hormone-sensitive motoneurons
 Androgens have been shown to exert a potent influence on the determination of sex differences in motoneuron number in the spinal nucleus of the bulbocavernosus (SNB), as well as on the retention of the perineal muscles innervated by these motoneurons. Females normally possess the perineal target muscles of the SNB at birth, but unless they are exposed to androgens, these muscles atrophy early in development. Androgens act in the establishment of sex differences in SNB motoneuron number by regulating normally-occurring motoneuron death. The death of motoneurons can be attenuated in females by treating them with androgens, resulting in a masculine motoneuron number. Conversely, males with a genetic deficiency in androgen receptors (testicular feminization mutation) also have a feminine number of SNB motoneurons, the result of a feminine pattern of motoneuron death.
Androgens have been shown to exert a potent influence on the determination of sex differences in motoneuron number in the spinal nucleus of the bulbocavernosus (SNB), as well as on the retention of the perineal muscles innervated by these motoneurons. Females normally possess the perineal target muscles of the SNB at birth, but unless they are exposed to androgens, these muscles atrophy early in development. Androgens act in the establishment of sex differences in SNB motoneuron number by regulating normally-occurring motoneuron death. The death of motoneurons can be attenuated in females by treating them with androgens, resulting in a masculine motoneuron number. Conversely, males with a genetic deficiency in androgen receptors (testicular feminization mutation) also have a feminine number of SNB motoneurons, the result of a feminine pattern of motoneuron death.
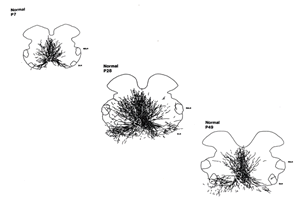 Motoneuron number is not the only developmental process regulated by gonadal steroids. During normal development, SNB dendrites grow exuberantly to almost twice their adult lengths by four weeks of age and subsequently retract, reaching their mature lengths by seven weeks of age. Like other aspects of SNB development, this dendritic growth is androgen-dependent. SNB dendrites in males castrated at one week of age fail to grow. SNB dendrites in castrates that receive testosterone replacement grow exuberantly, and remain exuberant into adulthood.
Motoneuron number is not the only developmental process regulated by gonadal steroids. During normal development, SNB dendrites grow exuberantly to almost twice their adult lengths by four weeks of age and subsequently retract, reaching their mature lengths by seven weeks of age. Like other aspects of SNB development, this dendritic growth is androgen-dependent. SNB dendrites in males castrated at one week of age fail to grow. SNB dendrites in castrates that receive testosterone replacement grow exuberantly, and remain exuberant into adulthood.
In the rat brain, estradiol is considered to be responsible for the differentiation of sexually dimorphic brain regions. In contrast, the development of sexually dimorphic nuclei in the rat spinal cord has been considered to be regulated by androgens and not estradiol. However, evidence obtained by our laboratory makes it clear that estradiol is in fact critically involved in spinal cord masculinization, thus unifying the process of masculinization across the mammalian central nervous system. Adminis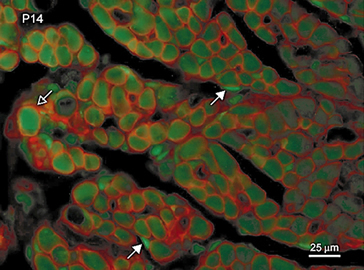 tration of estradiol to castrated males is as effective as dihydrotestosterone in supporting dendritic growth, and combined treatment with both metabolites is as effective as testosterone in fully supporting dendritic growth. Furthermore, blocking estradiol synthesis in intact males during this period results in significant reductions in SNB dendritic growth. These effects of estradiol appear to mediated through the neuromuscular periphery, acting at transiently expressed estrogen receptors in the target musculature. This transient receptor expression establishes the basis for the critical period for estrogenic support of SNB dendritic growth.
tration of estradiol to castrated males is as effective as dihydrotestosterone in supporting dendritic growth, and combined treatment with both metabolites is as effective as testosterone in fully supporting dendritic growth. Furthermore, blocking estradiol synthesis in intact males during this period results in significant reductions in SNB dendritic growth. These effects of estradiol appear to mediated through the neuromuscular periphery, acting at transiently expressed estrogen receptors in the target musculature. This transient receptor expression establishes the basis for the critical period for estrogenic support of SNB dendritic growth.
Estradiol continues to influence the mature functioning of this sexually dimorphic neuromuscular system in adulthood, apparently acting at least in part in the periphery. Assessing the roles of, and mechanisms regulated by, the critical forms of steroid hormones involved in sexual differentiation and function of the nervous system, and potential differences in their sites of action, is the focus of this project.